Insects・Others
APPLICATIONS
In vivo gene transfer into the adult honeybee brain by using electroporation
♦In vivo electroporation.
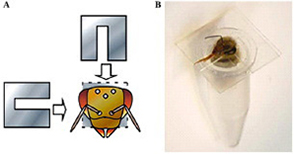
(A) The necks of honeybees that had settled in the tube were fixed in place by inserting two U-shaped plastic sheets for surgical ease and later release.
(B) A honeybee settled in the tube with her neck fixed prior to the operation.
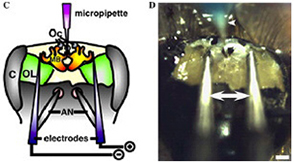
(C) Schematic representation of the micropipette and electrodes placed in the honeybee brain. Purple indicates micropipette filled with DNA solution, blue indicates electrodes. AN, antennae; C, compound eyes; MB, mushroom bodies; Oc, ocelli; OL, optic lobes.
(D) DNA injection and electroporation with needle electrodes.
Arrows and arrowhead indicate electrodes and micropipette for DNA injection, respectively. Scale bar, 300 µm.
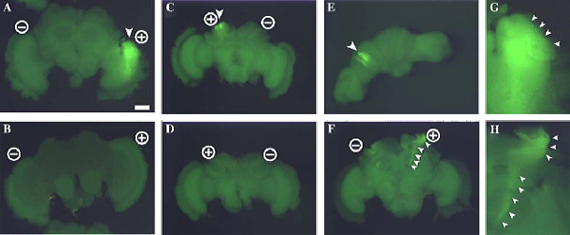
Fluorescent images of electroporated honeybee brain at 50 V, targeting the optic lobe (A, B) and mushroom bodies (C-F).
Fluorescent signals were detected around the anode position in the brain electroporated with GFP-expressing plasmid driven by the CMV promoter (A, C, E, F) and not in those electroporated without plasmid (B, D).
(G, H) Magnified views of GFP-expressing areas shown in (A) and (F), respectively.
Positions of electrodes are indicated by circles containing + or -.
Arrowheads indicate the regions in which fluorescence was detected (A, C, E, G) and neural projections from mushroom body cells (F, H).
Frontal view (A, B, F, G, H), rear view (C, D), and top view (E).
Panels (C) and (E) show different views of the same brain.
Takekazu Kunieda and Takeo Kubo, Department of Biological Sciences, Graduate School of Science, University of Tokyo
*University of Tokyo Patent Pending
*Biochemical and Biophysical Research Communications, Volume 318, Issue 1, Pages 25-31, May 21, 2004
PUBLICATIONS
- Xenopus
- Blastema
Hyperinnervation improves Xenopus laevis limb regeneration
Mitogawa K, Makanae A, Satoh A.
Dev Biol. 2018 Jan 15;433(2):276-286.
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescent Staining
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia tissue sections
- Avian Tissue Sections
- Plant tissue sections
Single-Cell/Micro-Particle Transfer
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, etc.
■ Animal Husbandry
- Sperm, embryos, tissue, etc.