Transfection into Mouse/Rat Embryos In Utero by Electroporation
Transfection into embryos: brain, spinal cord, inner ear and more.
APPLICATIONS
Gene transfer into embryonic brains using in utero electroporation technique
1) Materials
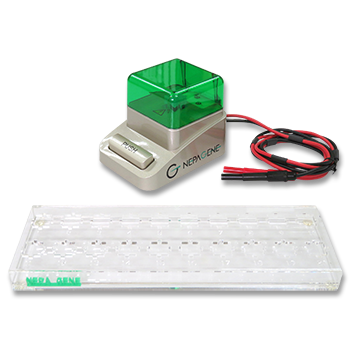
- In Vivo Electroporator: NEPA21/CUY21 SC/CUY21 EDIT (Nepa Gene Co., Ltd.)
- In Vivo Electrode: CUY650P3/CUY650P5 (Tweezers w/3mm or 5mm diameter platinum disk electrode, Nepa Gene Co., Ltd.)
- Aspirator tube assembly (Drummond)
- Optical fiber light (Technolight, Kenko, #KTS-100RSV)
- Sterile gauze (K-Pine, 7.5cm x 7.5cm)
- Surgical instruments: Fine forceps x 2, Surgery scissors x 2, Ring forceps, Needle holder, Surgical tape
- Nylon suture (Nesco, #HT1605NA75)
- Silk suture (D&G, #112451)

- Micropipette for DNA injection (make the micropipette by a micropipette puller)
2) In Utero Electroporation Procedure
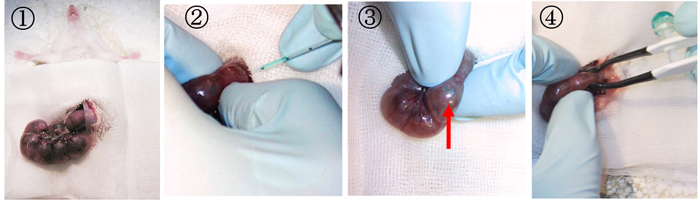
- A 2 cm midline incision is then made in the abdominal wall along the linea alba using a set of forceps and scissors. A piece of sterile gauze with a hole cut in the center is placed over the incision, and one uterine horn is drawn out through the hole in the gauze.
- After observing the orientation of the embryos through the wall of the uterine horn, a micropipette is inserted into the lateral ventricle, and 2-5μl of plasmid solution is injected by expiratory pressure using the aspirator tube assembly. When CAG-EGFP is used, a concentration of 1μg/μl is sufficient to visualize the migrating neurons.
- After the injection, DNA solution containing 0.01% FastGreen can be seen through the uterine wall (red arrow).
- After soaking the uterine horn with PBS, the head of embryo is pinched with a tweezers-type electrode, and electronic pulses are applied with the electroporator.
Electroporation settings for ICR mice
| Age | Electrode (diameter) | Voltage | Pulse On | Pulse Off | Number of Pulses |
| E12.5 | 3mm | 33V | 30msec | 970ms | 4 |
| E13.5 | 5mm | 30V | 50msec | 950ms | 4 |
| E14.5 | 5mm | 33V | 50msec | 950ms | 4 |
| E15- | 5mm | 35V | 50msec | 950ms | 4 |
If viability was prioritized over transfection efficiency, the number of pulses can be changed to two.
The actual current is displayed on the screen of the electroporator (NEPA21/CUY21/CUY21E, Nepa Gene). Make sure that the current would become 30-60mA. The current varies according to how the electrode applied or wetness of the uterine horn. Examine the gap between electrodes and the electrode contact areas to fit the current in the appropriate range. If the current is still above the range after the examination, change the voltage setting.
3) GFP expression
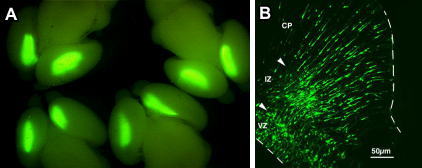
CAG-EGFP was injected into the both lateral ventricles of E14.5 mouse embryos and electronic pulses (33V, 50msec) were charged four times. 3 days later, the embryos (E17.5) were fixed and the brains were removed and examined under a fluorescence stereomicroscope (Fig. A).
Fluorescence was observed in the lateral region of the hemisphere onto which the anode had been placed and in the medial region of the opposite hemisphere.
Brains were frozen and sliced and the fluorescent image was obtained with a confocal laser microscope (Fig. B). GFP positive cells into which DNA was transferred at the ventricular zone (VZ) migrated to the intermediate zone (IZ) and cortical plate (CP). The arrowheads show the border between VZ and IZ and the border between IZ and CP. Dashed line show the border of tissues.
VZ: Ventricular Zone, IZ: Intermediate Zone, CP: Cortical Plate
Hidenori Tabata and Kazunori Nakajima, Department of Anatomy, Keio University School of Medicine
PUBLICATIONS
- E12.5_E13.0
- E14.0_E15.0
- E16.0_E17.0
- Cerebral_cortex
- Parietal_lobe_Somatosensory_area
Analyses of Conditional Knockout Mice for Pogz, a Gene Responsible for Neurodevelopmental Disorders in Excitatory and Inhibitory Neurons in the Brain
Hamada N, Nishijo T, Iwamoto I, Shifman S, Nagata KI.
Cells. 2024 Mar 19;13(6):540.
- E14.0
- Lateral_ventricle
Histological Analysis of a Mouse Model of the 22q11.2 Microdeletion Syndrome
Tabata H, Mori D, Matsuki T, Yoshizaki K, Asai M, Nakayama A, Ozaki N, Nagata KI.
Biomolecules. 2023 Apr 27;13(5):763.
- E14.0
- Cerebral_cortex
- Parietal_lobe_Somatosensory_area
Impaired Function of PLEKHG2, a Rho-Guanine Nucleotide-Exchange Factor, Disrupts Corticogenesis in Neurodevelopmental Phenotypes
Nishikawa M, Ito H, Tabata H, Ueda H, Nagata KI.
Cells. 2022 Feb 16;11(4):696.
- Lateral_ventricle
Simultaneous two-photon imaging of action potentials and subthreshold inputs in vivo
Bando Y, Wenzel M, Yuste R.
Nat Commun. 2021 Dec 10;12(1):7229
- E15.0
- Cerebral_cortex
- Neuroprogenitor_cells
IgSF11 homophilic adhesion proteins promote layer-specific synaptic assembly of the cortical interneuron subtype
Hayano Y, Ishino Y, Hyun JH, Orozco CG, Steinecke A, Potts E, Oisi Y, Thomas CI, Guerrero-Given D, Kim E, Kwon HB, Kamasawa N, Taniguchi H.
Sci Adv. 2021 Jul 14;7(29):eabf1600.
- E14.5
- Cerebral_cortex
- Neuroprogenitor_cells
Knockdown of Son, a mouse homologue of the ZTTK syndrome gene, causes neuronal migration defects and dendritic spine abnormalities
Ueda M, Matsuki T, Fukada M, Eda S, Toya A, Iio A, Tabata H, Nakayama A.
Mol Brain. 2020 May 24;13(1):80.
- E14.5_E15.5
- Lateral_ventricle
In Vivo Imaging of the Coupling Between Neuronal and CREB Activity in the Mouse Brain
Tal Laviv, Benjamin Scholl, Paula Parra-Bueno, Beth Foote, Chuqiu Zhang, Long Yan, Yuki Hayano, Jun Chu, Ryohei Yasuda
Neuron, 105 (5), 799-812.e5 2020 Mar 4
- E15.0
- Postnatal_electroporation
- P2
- Adult_Mouse_electroporation
- P21
Primary cilium-dependent cAMP/PKA signaling at the centrosome regulates neuronal migration
Stoufflet J, Chaulet M, Doulazmi M, Fouquet C, Dubacq C, Métin C, Schneider-Maunoury S, Trembleau A, Vincent P, Caillé I.
Sci Adv. 2020 Sep 2;6(36):eaba3992
- Embryonic_inner_ear
- Otocyst
Role of Dach1 Revealed Using a Novel Inner Ear-Specific Dach1-knockdown Mouse Model
Toru Miwa, Ryosei Minoda, Yoshihide Ishikawa, Tomohito Kajii, Yorihisa Orita, Takahiro Ohyama
Biol Open, 8 (8) 2019 Aug 20
- E14.5
- Cerebral_ventricles
In Vivo Single-Cell Genotyping of Mouse Cortical Neurons Transfected With CRISPR/Cas9
Steinecke A, Kurabayashi N, Hayano Y, Ishino Y, Taniguchi H.
Cell Rep, 28 (2), 325-331.e4 2019 Jul 9
- E13.5
- Cerebral_cortex
- Somatosensory_cortex
Pathological mTOR Mutations Impact Cortical Development
Tarkowski B, Kuchcinska K, Blazejczyk M, Jaworski J.
Hum Mol Genet. 2019 Jul 1;28(13):2107-2119.
- E15
- Cerebral_cortex
- Glucocorticoid_receptor
Persistence of Learning-Induced Synapses Depends on Neurotrophic Priming of Glucocorticoid Receptors
Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F.
Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13097-13106.
- E12.5
- E15.5
- E16
- Cortical_progenitors
Intersectional Monosynaptic Tracing for Dissecting Subtype-Specific Organization of GABAergic Interneuron Inputs
Yetman MJ, Washburn E, Hyun JH, Osakada F, Hayano Y, Zeng H, Callaway EM, Kwon HB, Taniguchi H.
Nat Neurosci. 2019 Mar;22(3):492-502.
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescence Quenching / in situ Hybridization Chain Reaction
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia Tissue Sections
- Avian Tissue Sections
- Plant Tissue Sections
- Chordate Tissue Sections
■ in situ HCR
- Detection of Target mRNA
Single-Cell/Micro-Particle Transfer
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, and more
■ Animal Husbandry
- Sperm, embryos, tissues, and more