Transfection into Mouse/Rat Zygotes by Electroporation (TAKE method)
APPLICATIONS
Introduction of ZFN, TALEN, and CRISPR-Cas into mammalian fertilized eggs by electroporation
Key Points of this Research Results
- We succeeded for the first time in the world in producing genetically modified animals (rats) using electroporation.
- We successfully introduced ZFN, TALEN, and CRISPR-Cas, which have attracted attention in recent years from researchers worldwide, into zygotes, and this method can be used as a technology to accelerate the genome editing technology.
- The electroporation method is easier to operate than the microinjection method, and animals with the target gene modified can be easily produced at research institutes and laboratories where skilled workers are not available.
- It can be applied to the introduction of genes into zygotes of many animal species, allowing the preparation of genetically modified animals in a short period of time using animal species suitable for research, thereby improving research efficiency (knockout and knock-in of mice and rats have also been successfully performed).
- This technique is named Technique for Animal Knockout system by Electroporation (TAKE method).
Result
The target gene was the interleukin 2 receptor gamma chain (Il2rg) gene, the causative gene of X-linked severe combined immunodeficiency (X-SCID). mRNA was prepared from ZFN, TALEN, and CRISPR-Cas. A CUY520P5 petri dish electrode connected to NEPA21 was filled with a solution of mRNA mixture, and the zygotes were placed in it for mRNA introduction by electroporation using the TAKE method. The zygotes were then transplanted into the female parents and raised to litter. As a result, we succeeded in obtaining offspring in which the target genes were disrupted in all cases of ZFN, TALEN, and CRISPR-Cas.
Experiment details
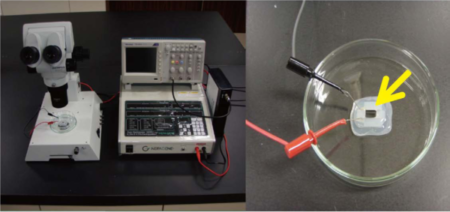
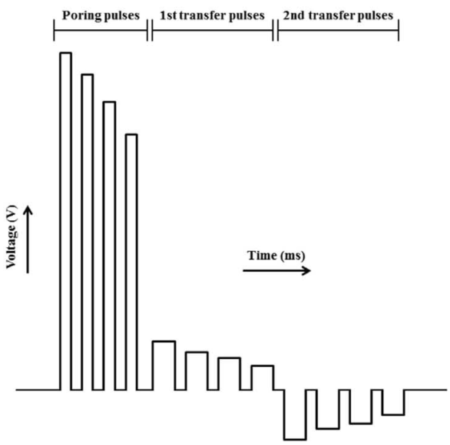
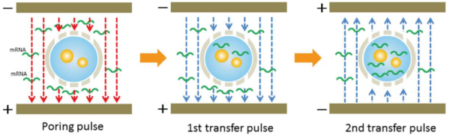

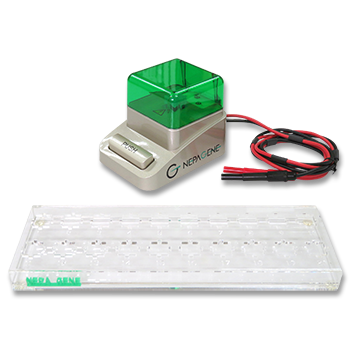
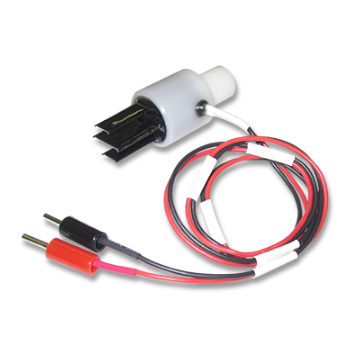
In this study, NEPA21 Super Electroporator (Picture A, Nepa Gene Co., Ltd.) and CUY520P5 Bath w/platinum plate electrodes on petridish, 5mm gap, (Picture B, Nepa Gene Co., Ltd.) were used to set up the 3-step electroporation (Figure C), where cells were punctured by the first-step electric pulse, and the second and the third step of the electrical pulse to introduce the gene into the zygotes in a stepwise manner. (Figure D)
Tetramethylrhodamine-labeled dextran (3 kDa, easy to visualize and non-cytotoxic) was used to examine the conditions for introducing foreign material into zygotes by electroporation. Zygotes in the pronuclear phase were collected from superovulated female rats one day after mating. Tetramethylrhodamine-labeled dextran was electroporated into rat zygotes with pulse widths of: 0 ms (control), 0.5 ms, 1.5 ms, and 2.5 ms. Dextran was introduced throughout the cytoplasm of the zygotes. (Picture E)
Prospects
Until now, the creation of genetically modified animals has required delicate and skilled techniques, and this has hindered the progress of research. The TAKE method developed in this study has made it possible to create animals with the desired gene modification in a short period of time with ease. The results of this research will greatly contribute to the acceleration of research requiring genetically modified animals, and it is expected that the method will be successfully applied to other animal species in the future.
Courtesy of Dr. Takehito Kaneko and Dr. Tomoji Mashimo, Institute of Laboratory Animals, Graduate School of Medicine, Kyoto University
Courtesy of Dr. Tetsushi Sakuma and Dr. Takashi Yamamoto, Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University
PUBLICATIONS
- KO_mice
- KI_mice
- KO_rats
- KI_rats
Genome Editing in Mouse and Rat by Electroporation
Kaneko T
Methods Mol Biol, 1630, 81-89 2017
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescence Quenching / in situ Hybridization Chain Reaction
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia Tissue Sections
- Avian Tissue Sections
- Plant Tissue Sections
- Chordate Tissue Sections
■ in situ HCR
- Detection of Target mRNA
Single-Cell/Micro-Particle Transfer
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, and more
■ Animal Husbandry
- Sperm, embryos, tissues, and more