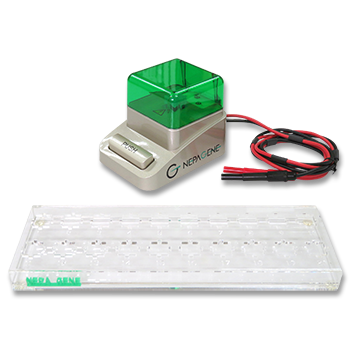
Production of Tetraploid Embryos by Electrofusion.
Tetraploid embryo complementation -two-cell embryos for ES-cell-derived mice
APPLICATIONS
Reprogramming of a melanoma genome by nuclear transplantation
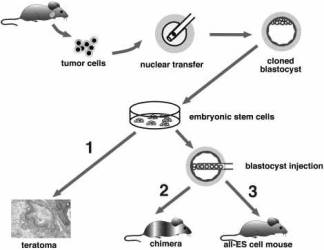
Fig. 1. Two-step cloning procedure to produce mice from cancer cells.
Two-step cloning procedure to produce mice from cancer cells.
Different tumor cells were used as donors for nuclear transfer into enucleated oocytes.
Resultant blastocysts were explanted in culture to produce ES cell lines.
The tumorigenic and differentiation potential of these ES cells was assayed in vitro by inducing teratomas in SCID mice (1), and in vivo by injecting cells into diploid (2) or tetraploid (3) blastocysts to generate chimeras and entirely ES-cell-derived mice, respectively.
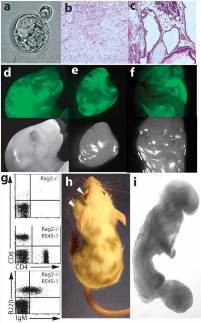
Fig. 2. Analysis of the developmental potential of R545-1 ES cells.
(a) A hatching blastocyst derived from a breast cancer cell by nuclear transfer shows a blastocoel cavity, trophectoderm layer, and an inner cell mass.
(b, c) H&E staining of teratoma sections produced from R545-1 ES cells shows differentiation into mature neurons, mesenchymal cells, and squamous epithelium (b), and columnar epithelium, chondrocytes, and adipocytes(c).
(d-f) Contribution of GFP-labeled R545-1 ES cells to newborn chimeras. Shown on top are the GFP images of the head (d), heart (e), and intestine (f) of one chimera. Below are the same images under phase contrast.
(g) FACS analysis of peripheral blood of a Rag2/R545-1 ES cell chimera shows the presence of B cells using antibodies FITC-IgM/PE-B220 and T cells using antibodies FITC-CD4/PE-CD8.
(h) Contribution of R545-1 cells to the skin indicates differentiation into melanocytes. Arrows depict spontaneous development of tumors on the eye and neck of chimera.
(i) Embryos produced entirely from ES cells by tetraploid complementation develop to E9.5 with obvious tail and limb buds, a closed neural tube, and a beating heart.
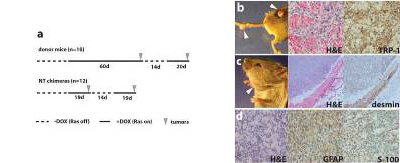
Fig. 3. Cancer phenotype in chimeric mice.
(a) Comparison of the average latency period of tumor development in the melanoma donor mice (top) with that in nuclear transfer (NT) chimeras (bottom). Note the similar latency of tumor development in NT chimeras with that in donor mice after readministration of doxycycline (recurrent tumors).
(b-d) Representative pictures and immunohistochemistry of tumors that formed in R545-1 NT chimeras. Arrows indicate sites of tumor growth. Melanomas (b), a rhabdomyosarcoma (c), and a malignant peripheral nerve sheath tumor (MPNST; d) were identified by H&E staining and immunohistochemistry with melanocyte-specific TRP-1 or muscle-specific desmin or MPNST-detecting GFAP and S-100 antibodies, respectively.
Hochedlinger K et al.,
Whitehead Institute for Biomedical Research, and Department of Biology,
Massachusetts Institute of Technology Genes Dev. 2004 Aug 1;18(15):1875-85.
PUBLICATIONS
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescent Staining
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia tissue sections
- Avian Tissue Sections
- Plant tissue sections
Single-Cell/Micro-Particle Transfer
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, etc.
■ Animal Husbandry
- Sperm, embryos, tissue, etc.