In Vivo Transfection into Mouse/Rat Muscle, Skin, Joint, Cartilage, Tumor and Others by Electroporation
APPLICATIONS
CRISPR/Cas9 transfection into mouse muscle by in vivo electroporation
Establishment of CRISPR gene repair evaluation system using eGFP KO mice
Figure 1: eGFP gene repair in eGFP KO mouse muscle.
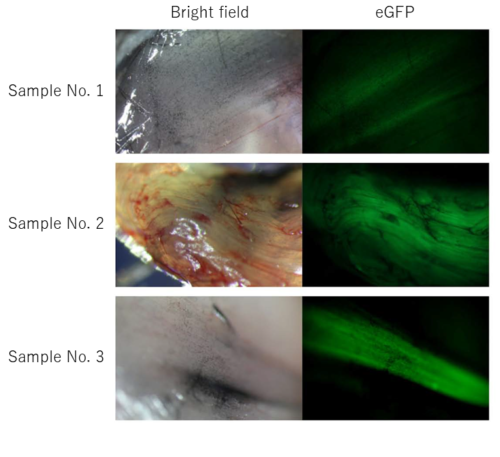
eGFP fluorescence in eGFP KO mouse muscle with attempted eGFP gene repair. Samples No. 1-3 are independent individuals.
To establish an in vivo gene repair evaluation system in muscle, we first investigated transfection conditions using an experimental system in which eGFP expression plasmids were introduced into wild-type mice.
As a result, we found that eGFP fluorescence could be confirmed in muscle with relatively good reproducibility by electroporation with the NEPA21 (Nepa Gene Co, Ltd.), followed by injection of a nucleic acid solution (100 µl) at a depth of 1.5 to 3.5 mm from the epidermis using a CUY568-4-0.5 needle array electrode (Nepa Gene Co., Ltd.), 30 minutes after administration of hyaluronidase.
Next, we examined whether the eGFP gene is repaired in the muscle of eGFP KO mice by direct transfection of CRISPR/Cas9-related nucleic acid.
We found that green fluorescence was observed in the muscles of all individuals, although the extent of fluorescence varied among individuals (Figure 1).
In courtesy of Dr. Hiromi Miura、 Department of Molecular Life Sciences, Basic Medical Science and Molecular Medicine, Tokai University School of Medicine
The Uehara Memorial Foundation Research Report (Volume 31, 2017)
Gene transfer into muscle by In Vivo electroporation
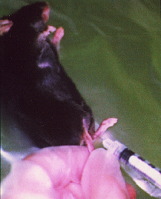
Injection of plasmid DNA into muscle
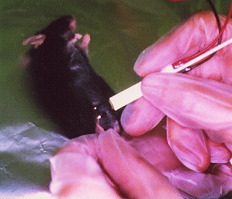
In Vivo Eletroporation
Electric pulses were delivered using an electric pulse generator (NEPA21 or CUY21; Nepa Gene Co.,Ltd.). Electrodes consisted of a pair of stainless steel needles of 5 mm in length and 0.4 mm in diameter, fixed with a distance (gap) between them of 3 mm or 5 mm (Nepa Gene Co.,Ltd.)
Protocol
Intramuscular DNA Injection
Anesthetize mice by intraperitoneal injection of 0.01 ml/g body weight of 6 mg/ml pentobarbital sodium solution. Inject the tibialis anterior muscles with 50 µg of purified closed circular DNA of pCAGGS-lacZ plasmid at 1.5 µg/µl in PBS using an insulin syringe with a 27-gauge needle.
Electroporation In Vivo
Insert a pair of electrode needles into the muscle to a depth of 5 mm to encompass the DNA injection sites. Deliver electric pulses using an electric pulse generator. Three 50-msec-long pulses of the indicated voltage (50-100 V) followed by three more pulses of the opposite polarity are administered to each injection site at a rate of one pulse per sec.
β-galactosidase Expression
Five days after DNA transfer, the expression of the lacZ gene is visualized by X-gal staining for -galactosidase activity
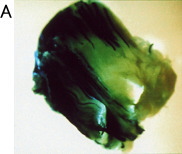
Whole muscle with electroporation
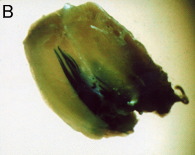
Whole muscle without electroporation
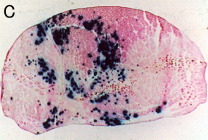
Transverse section with electroporation
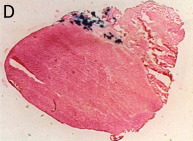
Transverse section without electroporation
X-gal Staining
The tibialis anterior muscles were fixed in cold 4% paraformaldehyde in PBS for 3 h, then washed in PBS for 1 h, and stained at 37°C for 18 h in the presence of 1mM X-gal to detect E. coli β-galactosidase activity.
For transverse sections, muscles were embedded in O.C.T. compound and frozen in dry ice-acetone.
Serial sections (15μm thick) were sliced with a cryostat and placed on slide glasses coated with 3-amino-propyltriethoxysilane.
The slices were fixed in 1.5% glutaraldehyde for 10 min at room temperature and then washed three times in PBS.
Samples were then incubated at 37°C for 3 h in the presence of 1 mM X-gal.
The muscle sections were counterstained with eosin. The control muscle (without electropulsation) showed only a small number of stained muscle fibers.
Electroporation increased both the number of positively stained muscle fibers and the density of staining.
Jun-ichi Miyazaki, Division of Stem Cell Regulation Research, G6 Osaka University Medical School
*Nature Biotechnology, Volume 16, Number 9, Pages 867-870, September 1998
Epidermis-Targeted Gene Transfer Using In Vivo Electroporation


1: Tweezers w/Variable Gap 3 Needle Fork & Stainless Steel Rectangle Plate Electrode, 5mm x 10mm (CUY663-5X10: NEPA GENE)
2: Pulse generator (CUY21 EDIT Square Wave Electroporator: NEPA GENE).
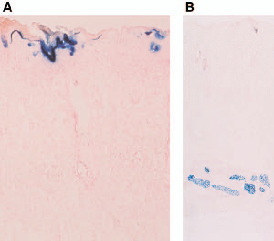
beta-Galactosidase expression on d 1 (A) and d 7 (B) after the pCAGGS-lacZ transfer with electroporation at 18V.
beta-Galactosidase was expressed in the upper most cell layers (horny, granular, and prickle cell layers) of the epidermis on d 1 (A), and in the subcutaneous muscle layer on d 7 (B).
Magnification: (A) x 250, (B) x 70
Hiroki Maruyama, Division of Clinical Nephrology and Rheumatology, Niigata University Graduate School of Medical and Dental Sciences
*Epidermal Cells Methods and Protocols, Series: Methods in Molecular Biology, Volume 289, Pages 431-436, October 2004
PUBLICATIONS
- Mice_tibialis_anterior(TA)_muscles
CUY650P5
Cooling-induced changes in intracellular hydrogen peroxide and gene expression in mouse skeletal muscle in vivo
Kano R, Takeda R, Sotani Y, Takagi R, Tabuchi A, Shirakawa H, Poole DC, Kano Y, Hoshino D.
Am J Physiol Regul Integr Comp Physiol. 2025 Jun 1;328(6):R758-R766.
- Mice_tibialis_anterior(TA)_muscles
CUY650P5
Exploring the Fate of Antibody-Encoding pDNA after Intramuscular Electroporation in Mice
Cuypers ML, Geukens N, Hollevoet K, Declerck P, Dewilde M.
Pharmaceutics. 2023 Apr 6;15(4):1160.
- Mice_tibialis_anterior(TA)_muscles
- Mice_flexor_digitorum_brevis(FDB)_muscles
Ambra1 deficiency impairs mitophagy in skeletal muscle
Gambarotto L, Metti S, Chrisam M, Cerqua C, Sabatelli P, Armani A, Zanon C, Spizzotin M, Castagnaro S, Strappazzon F, Grumati P, Cescon M, Braghetta P, Trevisson E, Cecconi F, Bonaldo P.
J Cachexia Sarcopenia Muscle. 2022 Aug;13(4):2211-2224.
- Mice_tibialis_anterior(TA)_muscles
- Mice_soleus_muscles(SOL)
CUY650P3
Gene Expression Profile at the Motor Endplate of the Neuromuscular Junction of Fast-Twitch Muscle
Huang K, Li J, Ito M, Takeda JI, Ohkawara B, Ogi T, Masuda A, Ohno K.
Front Mol Neurosci. 2020 Sep 8;13:154.
- Rat_external_auditory_canal
Keratinocyte Growth Factor (KGF) Induces Stem/Progenitor Cell Growth in Middle Ear Mucosa
Yamamoto-Fukuda T, Akiyama N, Kojima H.
Int J Pediatr Otorhinolaryngol, 128, 109699 Jan 2020
- Mice_tibialis_anterior(TA)_muscles
Fibroblast Growth Factor 21 Controls Mitophagy and Muscle Mass
Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V.
J Cachexia Sarcopenia Muscle, 10 (3), 630-642 Jun 2019
- Mice_tibialis_anterior(TA)_muscles
Key Amino Acid Substitution for Infection-Enhancing Activity-Free Designer Dengue Vaccines
Yamanaka A, Konishi E.
iScience. 2019 Mar 29;13:125-137.
- Mice_gastrocnemius_muscles
Involvement of the Protein Kinase Akt2 in Insulin-Stimulated Rac1 Activation Leading to Glucose Uptake in Mouse Skeletal Muscle
Takenaka N, Araki N, Satoh T.
PLoS One, 14 (2), e0212219 2019 Feb 8 eCollection 2019
- Mice_Extensor_digitorum_longus(EDL)_muscles
- Mice_tibialis_anterior(TA)_muscles
IGFN1_v1 Is Required for Myoblast Fusion and Differentiation
Li X, Baker J, Cracknell T, Haynes AR, Blanco G.
PLoS One, 12 (6), e0180217 2017 Jun 30 eCollection 2017
- Rat_external_auditory_canal
In Vivo Over-Expression of KGF Mimic Human Middle Ear Cholesteatoma
Yamamoto-Fukuda T, Akiyama N, Shibata Y, Takahashi H, Ikeda T, Koji T.
Eur Arch Otorhinolaryngol. 2015 Oct;272(10):2689-96.
- Rabbits_quadriceps_muscles
Production of research-grade antibody by in vivo electroporation of DNA-encoding target protein
Okahara F, Itoh K, Ebihara M, Kobayashi M, Maruyama H, Kanaho Y, Maehama T.
Anal Biochem. 2005 Jan 1;336(1):138-40.
- Mice_tibialis_anterior(TA)_muscles
Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy
Xue F, Takahara T, Yata Y, Kuwabara Y, Shinno E, Nonome K, Minemura M, Takahara S, Li X, Yamato E, Watanabe A.
Gut. 2003 May;52(5):694-700.
- Mice_tibialis_anterior(TA)_muscles
- Mice_skeletal_muscles
Attenuated acute liver injury in mice by naked hepatocyte growth factor gene transfer into skeletal muscle with electroporation
Xue F, Takahara T, Yata Y, Minemura M, Morioka CY, Takahara S, Yamato E, Dono K, Watanabe A.
Gut. 2002 Apr;50(4):558-62.
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescence Quenching / in situ Hybridization Chain Reaction
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia Tissue Sections
- Avian Tissue Sections
- Plant Tissue Sections
- Chordate Tissue Sections
■ in situ HCR
- Detection of Target mRNA
Single-Cell/Micro-Particle Transfer
■ マイクロピック&プレースシステム
- Picking and placing micro targets
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, and more
■ Animal Husbandry
- Sperm, embryos, tissues, and more