Transformation into E. coli and Bacteria by Electroporation
APPLICATIONS
– Transformation data of bacteria (E. coli) –
We measured gene transfer efficiency using the ELEPO21 in Gram-negative bacteria. Competent cells were prepared as usual from E. coli DH5α. The competent cells were mixed with pUC19 DNA, and a 20μl aliquot (containing 10^9-10^11 cells and 10 pg DNA in 10% glycerol solution) was transferred to the 1 mm gap electrode cuvette (EC-001S, Nepa Gene). The cuvette was set in the chamber connected to the ELEPO21, and delivered 3-step pulses as described below. All steps were done on ice. After electroporation, the cells were plated on LB agarose medium containing ampicillin, and colonies formed were counted. Transformation efficiency was expressed as a number of colonies per ug plasmid DNA used.
[ELEPO21 pulsing conditions, Fig. 1]
Poring Pulse (voltage: 2,000 V, pulse length: 2.5 msec, pulse interval: 50 ms, number of pulses: 1, polarity: +)
Transfer Pulse (voltage: 150 V, pulse length: 50 msec, pulse interval: 50 ms, number of pulses: 5, polarity: +/-)
To evaluate the above results, we measured gene transfer efficiencies using a conventional electroporator (ECM630, BTX) that deliver a single exponential pulse as described below.
[ECM630 pulsing conditions, Fig. 2]
Single pulse (voltage: 2,000 V, resistance: 200 ohms, capacitance: 25 uF, number of pulse: 1)
| Fig. 1: ELEPO21 pulse shape | Fig. 2: ECM630 pulse shape | |
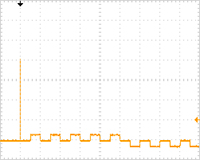 | 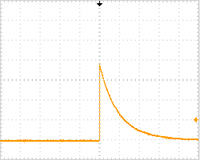 |
Fig. 3:
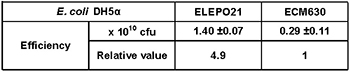
[Experimental results]:
The above cell suspensions (sample resistance value: 7.7 K ohms) were used for electroporation. The tranformation efficiency obtained by the ELEPO21 electroporator was approximately 5 times higher than that by the ECM630 electroporator (Fig. 3).
*The values are averages of repeated experiments.
*The optimum pulsing conditions were used for ELEPO21 and ECM630.
*cfu: colony forming unit.
PUBLICATIONS
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescence Quenching / in situ Hybridization Chain Reaction
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia Tissue Sections
- Avian Tissue Sections
- Plant Tissue Sections
- Chordate Tissue Sections
■ in situ HCR
- Detection of Target mRNA
Single-Cell/Micro-Particle Transfer
■ マイクロピック&プレースシステム
- Picking and placing micro targets
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, and more
■ Animal Husbandry
- Sperm, embryos, tissues, and more