Hybridoma Production
APPLICATIONS
Comparison of 3 cell fusion techniques using polyethylene glycol, electrofusion, and HVJ envelope vector for production of monoclonal antibody by iliac lymph node methods
Electrofusion (Electro Cell Fusion)
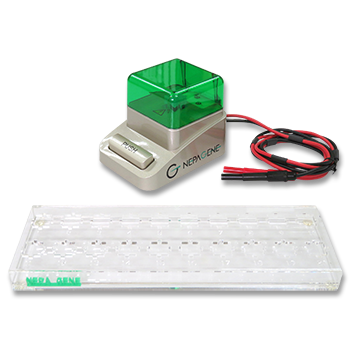
Electrofusion was performed using the ECFG21 or the LF201 Electro Cell Fusion Generator (Nepa Gene, Japan) and the CUY497P2 MS Stand Model Chamber Type Platinum Electrode, L80mm x W2mm x H5mm, 0.8ml (Nepa Gene, Japan), (Fig. 1). The electrofusion buffer was composed of 0.3M mannitol, 0.1mM calcium chloride and 0.1mM magnesium chloride.

1) Using a ratio of one-to-one, B lymphocytes from animals immunized with ovalbumin and myeloma cells were put into a 15ml centrifuge tube containing electrofusion buffer and centrifuged. The supernatant was removed and the cells were suspended with 2.4ml of electrofusion buffer solution.
2) Set the Generator as follows: AC (1MHz, 30Vrms, 20sec), DC (350V, pulse length: 30sec, pulse interval: 0.5sec, 3 times). Connect the CUY497P2 electrode.
Inject 0.8ml of the suspended cells into the CUY497P2 electrode. Press the start button and the AC current for 20 seconds will bring the cells into alignment (pearl chain), then the Generator will automatically apply DC pulses three times. Then remove cells from Electrode. Repeat the step 2 two more times.
3) Centrifuge for 5 minutes at 1,000 rpm. Mix cells with HAT medium add BM-Condimed H1 and inoculate into four 96 well plates.
Comparison of methods using PEG and electrofusion
Experiments using PEG and electrofusion were done with iliac lymph node lymphocytes from mice. The cells in two cryogenic vials were thawed and mixed (approx. 4 x 107 cells). Then half of the cells were fused by PEG and the other half of the cells were fused by electrofusion. This set of experiments was done three times. The result was that the number of positive wells by electrofusion was 1.5-4.0 times as many as by PEG (Table 1). The average rate was 2.8 times.
Table1: Comparison of fusion methods using mouse iliac lymph node lymphocytes
| PEG Positive Wells | Electrofusion Positive Wells | PEG: Electrofusion Ratio | |
| 1st | 166 | 250 | 1:1.5 |
| 2nd | 60 | 182 | 1:3.0 |
| 3rd | 65 | 262 | 1:4.0 |
Comparison of methods using PEG and electrofusion
Experiments using PEG and electrofusion were done with iliac lymph node lymphocytes from mice. The cells in two cryogenic vials were thawed and mixed (approx. 4 x 107 cells). Then half of the cells were fused by PEG and the other half of the cells were fused by electrofusion. This set of experiments was done three times. The result was that the number of positive wells by electrofusion was 1.5-4.0 times as many as by PEG (Table 1). The average rate was 2.8 times.
Discussion of electrofusion
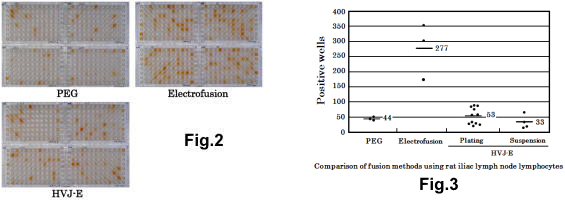
Cell fusion with PEG, electrofusion and HVJ-E was done using iliac lymph node lymphocytes from rats (Fig. 2 and 3). Cell fusion by electrofusion can be done with smaller number of lymphocytes than by PEG and HVJ-E, and its operation time is shorter than PEG and HVJ-E. The growth of fused cells after electrofusion was faster because its damage seemed to be low. Therefore ELISA screening for positive wells by electrofusion was done one day earlier than by PEG. The fusion technique does not vary from individual to individual because electrofusion operation is simple. Once the fusion operation and electric setting are optimized, the fusion with the equal condition can be always generated for the same animal’s lymphocytes. The fusion with an equal condition leads to small variation in the data. With electrofusion, the fusion time is short, the cell damage is low and the fusion efficiency is the highest. At this experiment, the number of positive wells was 6 times better compared to PEG.
◆ Summary
3 kinds of cell fusion methods were compared using iliac lymph node lymphocytes from mice and rats. PEG method is economical but the result varies according to fusion. The fusion efficiency by HVJ-E is the same or higher than PEG but it is better than PEG in that fused cells grow vigorously. The fusion efficiency by electrofusion is approx. 6 times better in the use of rat lymphocytes and approx. 3 times better in the use of mouse lymphocytes as high as by PEG. Electrofusion method is clearly the best out of the three in terms of efficiency, reproducibility, time and cost.
Satoko Inoue and Yoshikazu Sado, Division of Immunology, Shigei Medical Research Institute
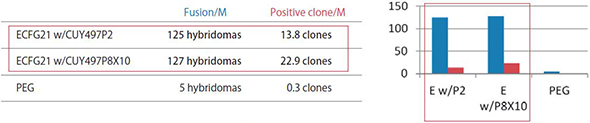
ECFG21 vs PEG Small Molecule Antigens or Peptide Antigens
The efficiency by the ECFG21 is 25-100 times higher than PEG.
These three antigens were all classified as difficult to obtain positive clones. The ECFG21 vastly increased the number of clones in all cases, and in at least one case, resulted in positive clones when none resulted with the traditional PEG method.
Fusion/M: Number of hybridomas generated per 1 x 10^6 lymphocytes
Positive clone/M: Number of clones producing monoclonal antibodies against the antigen per 1 x 10^6 lymphocytes
ECFG21 w/CUY497P2: Electrofusion performed using CUY497P2 electrode (for up to 5 x 10^7 lymphocytes)
ECFG21 w/CUY497P8X10: Electrofusion performed using CUY497P8X10 electrode (for large volumes: one-mouse lymphocytes)
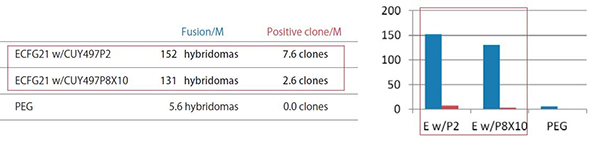
1) Antigen used: 20 kDa immunosuppressive protein with high homology
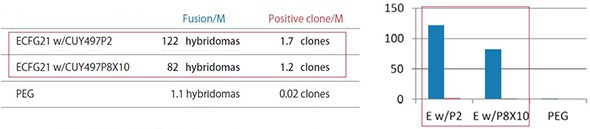
2) Antigen used: Hydrophobic octapeptide
3) Antigen used: Highly hydrophobic 17 amino acid peptide.
PUBLICATIONS
- Hybridoma
- Monoclonal_antibody
- Spleen
- lymphocytes
- mouse_myeloma_cells
Development of a monoclonal antibody to study MARCH6, an E3 ligase that regulates proteins that control lipid homeostasis
Xu S, Donnelly L, Kober DL, Mak M, Radhakrishnan A.
J Lipid Res. 2024 Sep 19:100650.
- Hybridoma
- Monoclonal_antibody
- Spleen
- lymphocytes
- mouse_myeloma_cells
Discovery of RXFP2 genetic association in resistant hypertensive men and RXFP2 antagonists for the treatment of resistant hypertension
Zhang SS, Larrabee L, Chang AH, Desai S, Sloan L, Wang X, Wu Y, Parvez N, Amaratunga K, Hartman AC, Whitnall A, Mason J, Barton NP, Chu AY, Davitte JM, Csakai AJ, Tibbetts CV, Tolbert AE, O'Keefe H, Polanco J, Foley J, Kmett C, Kehler J, Kozejova G, Wang F, Mayer AP, Koenig P, Foletti D, Pitts SJ, Schnackenberg CG.
Sci Rep. 2024 Jun 8;14(1):13209.
- Hybridoma
- Monoclonal_antibody
- mouse_myeloma_cell_line(NS-1)
- lymphocytes
Developing inhibitory peptides against SARS-CoV-2 envelope protein.
Bekdash R, Yoshida K, Nair MS, Qiu L, Ahdout J, Tsai HY, Uryu K, Soni RK, Huang Y, Ho DD, Yazawa M.
PLoS Biol. 2024 Mar 14;22(3):e3002522.
- Hybridoma
- Monoclonal_antibody
- Spleen
- Lymph_node
Establishment of a Monoclonal Antibody against Human NTCP That Blocks Hepatitis B Virus Infection
Takemori T, Sugimoto-Ishige A, Nishitsuji H, Futamura Y, Harada M, Kimura-Someya T, Matsumoto T, Honma T, Tanaka M, Yaguchi M, Isono K, Koseki H, Osada H, Miki D, Saito T, Tanaka T, Fukami T, Goto T, Shirouzu M, Shimotohno K, Chayama K.
J Virol. 2022 Mar 9;96(5):e0168621.
- Hybridoma
- Monoclonal_antibody
- Lymph_node
An improved iliac lymph node method for production of monoclonal antibodies
Kobayashi T, Namba M, Kohno M, Koyano T, Sado Y, Matsuyama M.
Dev Growth Differ. 2022 Jan;64(1):38-47.
- Hybridoma
- Monoclonal_antibody
- Lymph_node
Establishment of an antibody specific for AMIGO2 improves immunohistochemical evaluation of liver metastases and clinical outcomes in patients with colorectal cancer
Goto K, Osaki M, Izutsu R, Tanaka H, Sasaki R, Tanio A, Satofuka H, Kazuki Y, Yamamoto M, Kugoh H, Ito H, Oshimura M, Fujiwara Y, Okada F.
Diagn Pathol. 2022 Jan 30;17(1):16.
Electroporation
■ Cell Cultures
- Primary Cell Cultures
- Stem Cells
- Organoids
- Cell Lines
- Cells in Adherence
■ In Vivo Mice/Rats
- Zygotes In Vitro (TAKE method)
- Zygotes In Oviduct (i-GONAD method)
- Embryos In Utero
- Ex Utero Embryos In Vitro
- Brain
- Retina / Cornea / Spinal Cord / Sciatic Nerve
- Lung / Spleen / Liver / Stomach/ Kidney / Intestine
- Pancreas / Islets of langerhans
- Testis / Ovary / Prostate / Gonad / Uterus
- Muscle / Skin / Joint / Cartilage / Tumor / Others
■ In Vivo Other Animals
- Bovine/Porcine/Other Animal Zygotes
- Hamster Zygotes in Oviduct (i-GONAD method)
- Monkey Skin
- Chicken (In Ovo・Others)
- Zebrafish & Other Fishes
- Insects・Others
■ Plant Cells & Algae
- Plant Cells
- Algae
■ Exosomes
- Exosomes
■ Bacteria, Yeast, Fungi
- E. coli/Bacterial Cells
- Yeasts/Fungi
- Bacterial cells/Yeasts/Fungi (NEPA Porator)
Drug Delivery and Transfection
■ Ultrasound Transfection and Drug Delivery (Sonoporation/Fus)
- Brain
- Liver/Skin/Other Applications
- Heart
- Cell Culture
- Lung
- Muscle
Electro Cell Fusion
■ Hybridoma Production
- Monoclonal antibodies, etc
■ Oocyte Activation
- Electrical stimulation before/after Intracytoplasmic sperm injection (ICSI)
■ Somatic cell nuclear transfer (SCNT)・ Oocytes Nuclear Transfer
- Animal cloning
■ Tetraploid Embryos Production
- 2 Cell Embryos (Tetraploid)
■ Other Applications
- Liposome・Protoplast・Yeast, etc.
Fluorescent Staining
■ Autofluorescence Quenching
- Mammalian Tissue Sections
- Fish Tissue Sections
- Amphibia tissue sections
- Avian Tissue Sections
- Plant tissue sections
- Chordate tissue sections
Single-Cell/Micro-Particle Transfer
■ Micro targets
- Animal cells
■ Micro liquid
- Plant cells
Cell Freezing
■ Cell Therapy
- Stem cells, primary cells, and more
■ Animal Husbandry
- Sperm, embryos, tissues, and more